Chronic Wounds:
FGF-1 for Chronic Wounds
Zhittya Genesis Medicine is developing a drug treatment which, we believe, can effectively heal non-healing chronic wounds using FGF-1, such as diabetic foot ulcers, pressure sores, venous ulcers, and other non-healing wounds.
Non-healing chronic wounds are the result of a lack of blood flow to a particular area which has been damaged by a cut, scrape, or scratch. Without proper blood flow, the body is not able to repair that area, leading to non-healing wounds that are prone to infection, and without treatment, amputation.
In the late 1980s, Merck developed our drug, FGF-1, to treat non-healing Diabetic Foot Ulcers (DFU), a non-healing wound that affects ~20% of diabetics within their lifetimes. In US FDA clinical trials, Merck showed that these non-healing wounds were able to 100% heal in every patient suffering from a DFU.
Zhittya has since purchased the rights to Merck’s data and is continuing on the technology that Merck had developed for diabetic foot ulcers, and applying it to all chronic wounds. Zhittya Genesis Medicine’s administration has seen that FGF-1 can significantly accelerate the healing of chronic wounds, with studies reporting complete closure in all treated diabetic foot ulcers and more than doubled healing rates in venous leg ulcers, without serious adverse events.
Leading medical research institutions and Zhittya believe that Type-2 Diabetes is a neurological disease caused by the glucose sensing neurons in the brain not performing normally. We believe, our molecule, FGF-1, resets the glucose-sensing neurons in the brain.
Medical Research Study to Treat Non-Healing Chronic Wounds- Las Vegas, NV
Zhittya Genesis Medicine has received Institutional Review Board (IRB) approval to do a 100 person medical research study in Las Vegas, Nevada to reconfirm whether FGF-1 can treat non-healing chronic wounds.
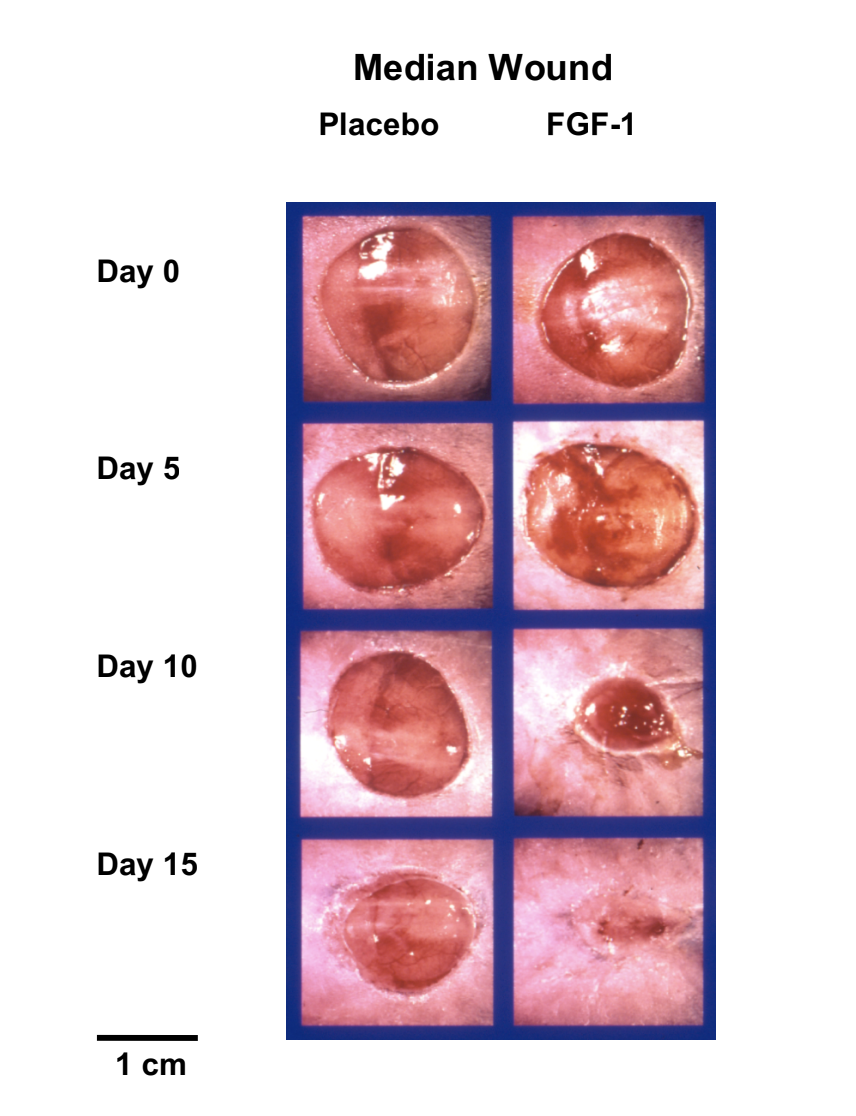
In this image above, you can see how FGF-1 is able to completely close non-healing wounds in a diabetic model after only 15 days of topical dosing, whereas the placebo group still has open wounds.
In this 45 minute presentation, you can see all of the data that supports our treatments of not just diabetic foot ulcers, but non-healing chronic wounds as a whole.
Register for Medical Research Study to Treat Non-Healing Chronic Wounds.
Located in: Las Vegas, Nevada
If you have a non-healing chronic wounds and are interested in potentially participating in our medical research study to treat chronic wounds, complete the form below to express your interest. For those patients that are selected to participate, they will bear no cost for participating in the study other than their own travel and accommodations. This is a voluntary medical research study to evaluate FGF-1's ability to potentially treat non-healing chronic wounds. We'll screen applicants for eligibility, but there's no guarantee of participation or specific results. All processes follow ethical guidelines, including informed consent.
Disclaimer: This form is for expressing interest in a medical research study only. It does not constitute enrollment, medical advice, or a guarantee of participation. All studies comply with FDA guidelines and ethical standards. Consult your physician before considering any research involvement.
Zhittya is contracting a fund of $5 million USD to fund this study to verify if FGF-1 can treat non-healing chronic wounds. Investors interested in funding the study have the potential profit returns of:
10x return from a 50% royalty on all FGF-1 revenues for Chronic Wounds OR
2x their investment (at investor put option) after 48 months, if the investor is not happy
Minimum: $25,000.
Submit the form for more details. This is informational only—not an offer to invest.
Information on Investing into Medical Research Studies for Chronic Wounds:
Disclaimer: This is not an offer to sell, nor a solicitation of an offer to buy, any securities. Any potential investment opportunities will be provided only to qualified, accredited investors in compliance with SEC regulations, including Rule 506(c) of Regulation D where applicable. Investments involve significant risk, including the potential loss of principal. Past performance is not indicative of future results. For details, accredited investors may request a private placement memorandum upon qualification. Zhittya Genesis Medicine makes no representations or warranties regarding returns or outcomes.
History
FGF-1 is a potent stimulator of angiogenesis, the growth of new blood vessels, and is capable of growing new blood vessels in ischemic areas of the body. US FDA Phase II clinical trials have shown that non-healing chronic wounds, such as diabetic foot ulcers, are able to rapidly accelerate the healing process and treat non-healing wounds, all with no known side effects in these trials.
The management of Zhittya has spent over $190 million dollars to advance this molecule bringing it as far as US FDA Phase IIA/Phase IIB clinical trials in various indications.
In the past, FGF-1 has been able to grow new blood vessels in the human body. In a US FDA Phase IIA clinical trial, conducted at the University of Cincinnati, our drug was able to grow new blood vessels in the hearts of individuals with coronary artery disease, improving many of their symptoms. Moreover, this drug has been studied in diabetic foot uclers, stroke recovery, and more.
Watch as Zhittya Genesis Medicine’s drug is used to grow new blood vessels in the human heart in a US FDA Phase IIA Clinical Trial done at the University of Cincinnati, and reported by ABC Nightly News. Zhittya believes that by growing new blood vessels in chronic wounds, that these wounds be closed up and treated.
News
Meet Zhittya’s Team
Our Partners